This product is designed for the qualitative test of the cell-free fetal DNA in the plasma of pregnant women who are pregnant with a single fetus, whose gestational age are between 8 weeks and 24 weeks, and whose risks of giving birth to a baby with chromosomal aneuploidies are high. By analyzing the differences
This PGS Detection Kit is used for the qualitative detection of embryonic cells obtained by intracytoplasmic sperm injection (ICSI), including whole-genome amplification products of blastomere cells (Day 3) or blastocyst trophoblast cells (Day 5). By analyzing the DNA sequence information in the single-cell whole genome amplification product, the difference in the number of chromosomes in
This kit is used for the in vitro qualitative detection of human whole blood genomic DNA sample, which can detect the commonly seen 3 kinds of deletion type α-thalassemia gene mutation (–SEA, -α3.7 and -α4.2), 3 kinds of non-deletion type α-thalassemia gene mutation (αCSα, αQSα and αWSα) and 17 kinds of β-thalassemia gene mutation (41-42M,
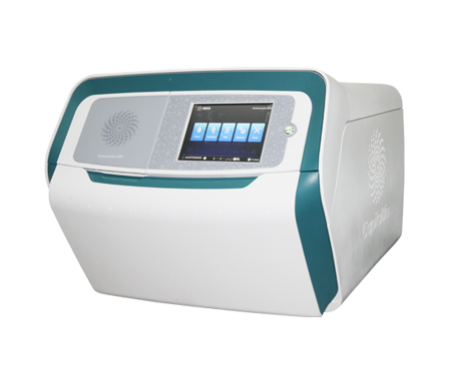
CapitalBio® BioelectronSeq 4000 System (abbreviation: BES 4000) is a next-generation sequencing system developed and produced by the cooperation between CapitalBio Corporation and Thermo Fisher Scientific Inc. in China. The system using semiconductor sequencing technology, combined with clinical testing kits and automated data analysis & management software, is suitable to be deployed and placed in clinical
- 1
- 2